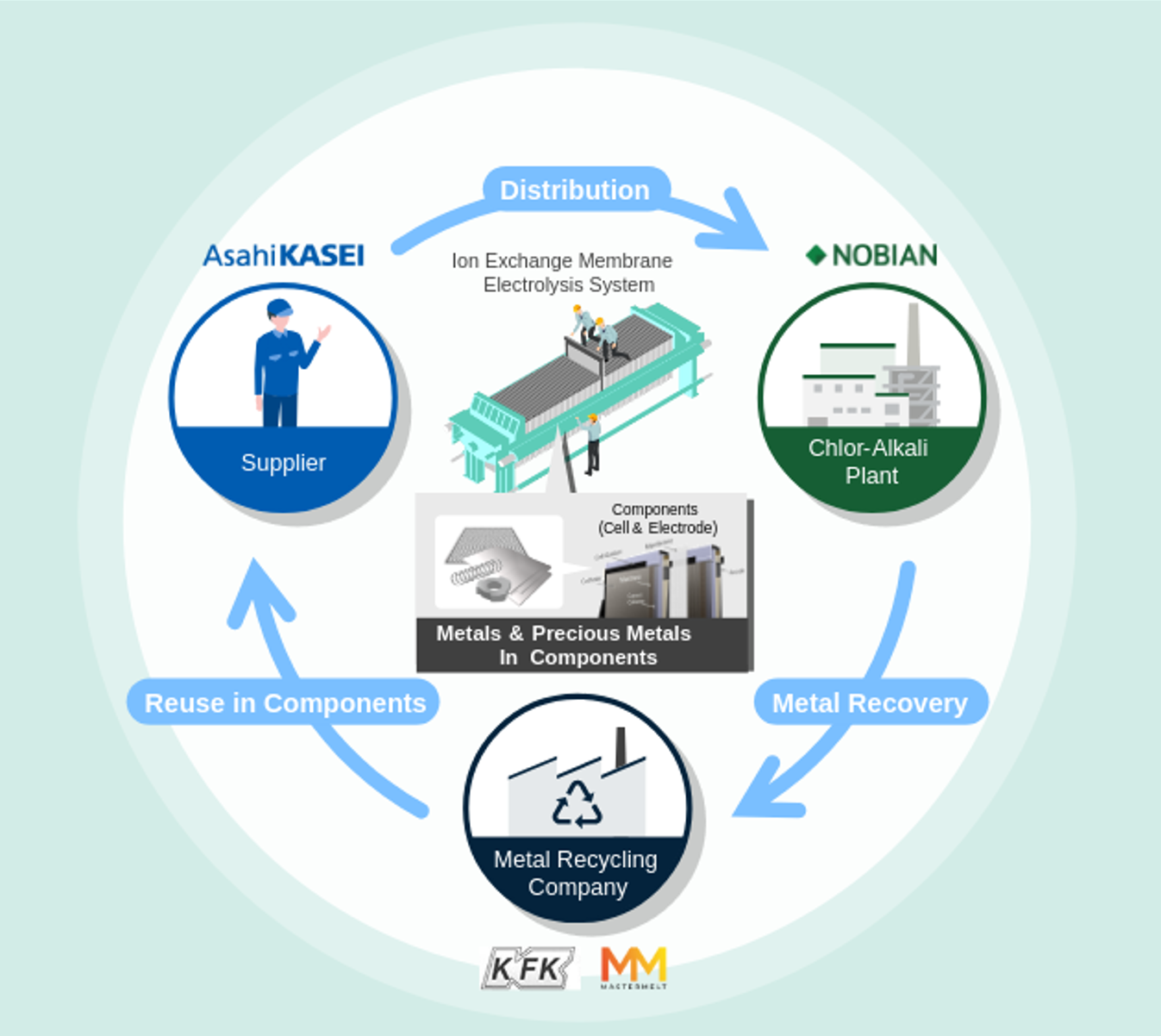
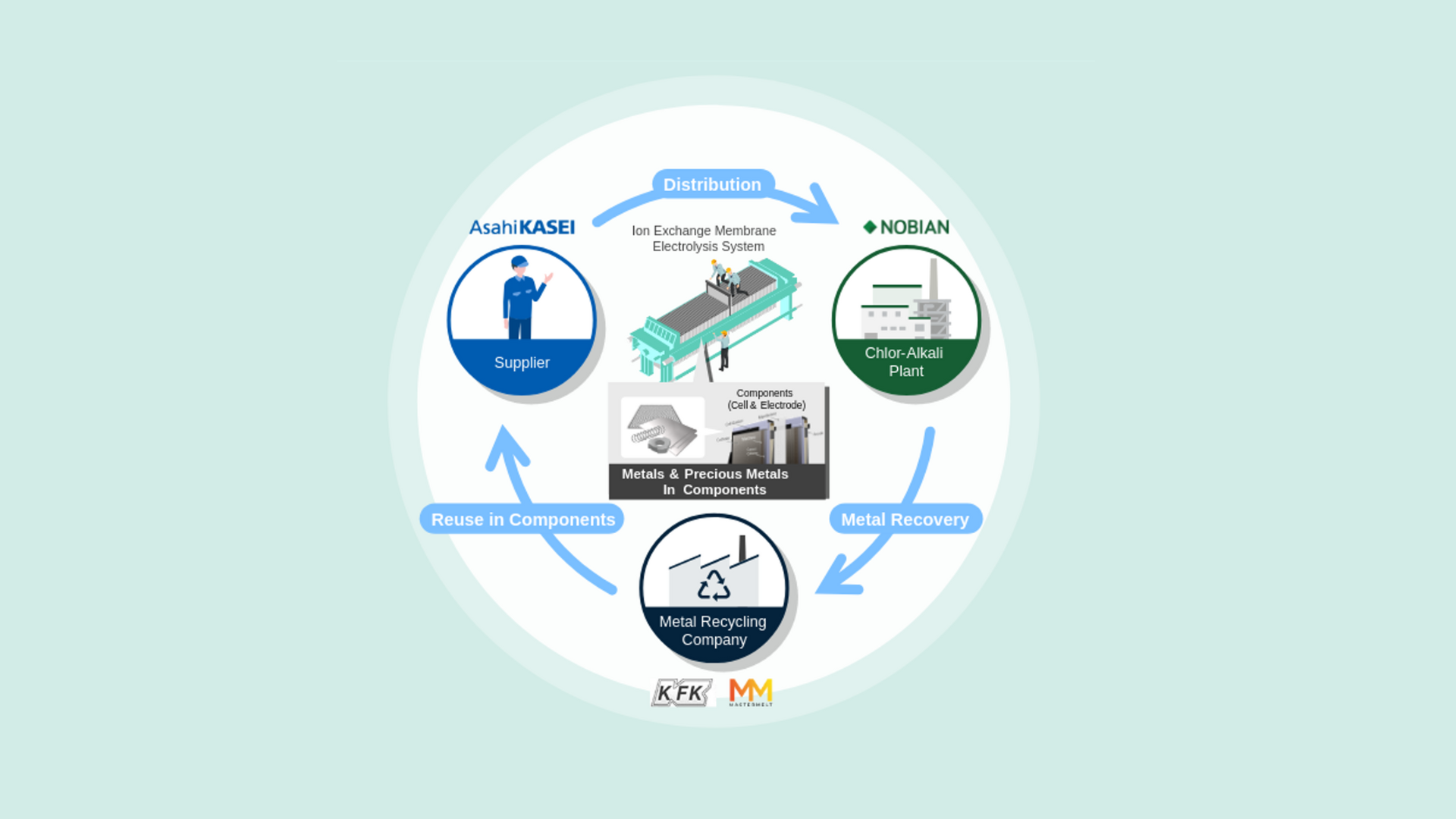
Electrolysis in the chlor-alkali industry
In the chlor-alkali industry, electrolyzers produce chlorine, caustic soda and hydrogen from brine by using electricity. These chemicals are crucial for everyday life and materials and are key components for the energy transition, such as windmill blades, batteries, and insulation materials. Important components used in electrolyzers require rare metals like iridium and ruthenium for the electrodes used in the process.
Due to the increasing demand for batteries and electrical components, as well as geopolitical developments, the costs of rare metals needed to manufacture electrolysis cells are rising. This trend is expected to continue and to accelerate, due to the growing need of electrolyzers for water electrolysis for hydrogen production. Consequently, the chlor-alkali industry faces a challenge to make efficient use of limited precious metal resources.
Creating a circular value chain
To address this challenge, Nobian and Asahi Kasei started a pilot project in Europe in 2023 as the first step towards realizing a circular economy for precious and other metals. While this pilot project focused on the reuse and reduction of existing cells, the new initiative focuses on the recovery of recycled metals from electrodes that have reached the end of their service life, refining them for reuse as raw materials for new electrodes.
Together Nobian and Asahi Kasei are working to create an ecosystem within the chlor-alkali industry that circulates limited rare and other metal resources as much as possible, ensuring a stable supply of electrodes for salt electrolysis, and ultimately, a stable supply of essential caustic soda and chlorine to the world.
Jacky Oonincx, Director Technology Chlor-Alkali & Chloromethanes at Nobian: ‘Our chlor-alkali electrolysis technology uses rare metals like ruthenium and iridium. In collaboration with Asahi Kasei Corporation, one of our main electrolyzer suppliers, we are developing methods to recycle these metals from electrodes. Most of the ruthenium and iridium can be recovered and, along with titanium and nickel, will be reused to produce new, recycled electrodes and cell frames, creating a circular value chain.’
Marco Waas, Chief Technology and Sustainability Officer at Nobian: ‘By securing these critical raw materials, we contribute to the EU’s ‘Critical Raw Material Act’ which requires a minimum of 25% of strategic raw materials, including iridium and ruthenium, to be recycled within the EU by 2030. This recycling initiative supports Nobian's Grow Greener Together sustainability approach, by reducing our environmental impact by minimizing the mining of these essential materials.’
Yoshifumi Kado, Senior General Manager of Ion Exchange Membrane & Electrolysis System Division at Asahi Kasei: ‘This initiative represents an important step forward for the chlor-alkali industry and furthers our commitment to advancing circular economy solutions. By combining the expertise of Asahi Kasei, Nobian, Furuya Metal, and Mastermelt, we will be able to garner even more value from the important precious metals used in electrolyzer technology while transforming sustainability goals into practical, scalable outcomes.’
Additional participants and future goals of the recycling initiative
In addition to Nobian and Asahi Kasei, Furuya Metal (Tokyo, Japan) and Mastermelt (London, UK) are also participating in the initiative. Furuya Metal specializes in industrial-use rare metals, whose integrated business ranges from refining to manufacturing and fabrication to recycling. Mastermelt leads in precious metal recycling and has expertise in recovering metals from industrial waste streams, jewellery, manufacturing scrap, and end-of-life products.
In the future, the recycling project aims to improve and track the circularity of the ecosystem, reduce CO₂ emissions across the supply chain by using recycled metals in cells and electrodes, and expand into the water electrolysis field.